


Next: About this document ...
PHYSICS 313
Midterm November 5 2003.
Answer all questions. Allowed
aids 1 double-sided formula sheet. Calculator.
Problem 1:
The entropy of one mol of argon at 298 K is
154.84 J/K.
a: Assume that argon is a monatomic ideal gas. Use the information provided to find the quantum volume at 298 K in
the Sackur Tetrode equation
b: What is the entropy of 1 mol of
Argon at 400 K, if the pressure remains 1 bar? You may assume that the quantum volume 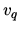 is
proportional to
is
proportional to 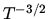 .
.
Problem 2:
A container has initially 2 compartments each with volume 1 liter. Each compartment contains 1 mol of the same monatomic ideal gas. The temperature of the two compartments are initially 300K and
400 K. The wall separating the two compartments is removed.
a: What is the final pressure and temperature?
b: What is the change in entropy when the gases in the two containers are mixed?
c: Would you have got a different result in b: if the gas had been diatomic?
Problem 3:
a: The pressure of 1 mol of liquid water is increased isothermally from 1 bar to 300 bar
at T=298 K. Estimate the change in the Gibbs free energy neglecting the change in volume.
b: The pressure 1.5 mol of an ideal gas is increased isothermally from 1 bar to 300 bar. Estimate the change in the Gibbs free energy if the temperature is 298 K.
(now you cannot neglect the change in volume).
c: In the electrolysis of water
2 electrons pass through the circuit per formula unit.
Will the minimum voltage required increase or decrease if the
electrolysis takes place under high pressure? Justify your answer.
Some
formulas
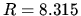 J mol
J mol K
K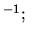
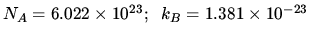 J K
J K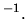
1 atm 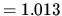 bar
bar
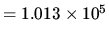 N m
N m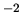
Ideal gas:
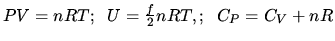
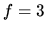 for monatomic gas;
for monatomic gas; 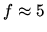 for diatomic gas
for diatomic gas
The density of liquid water is 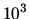 kg m
kg m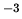 . The molecular weight
of water is 18 g mol
. The molecular weight
of water is 18 g mol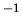 .
.



Next: About this document ...
Birger Bergersen
2003-11-12