

Next: About this document ...
PHYSICS 313
Optional supplementary problems
If you have missed one or more of the regular assignments you can
hand in solutions to this problem set for marking, but do so before class
Wednesday November 29.
1:Derivation of Equation of State.
In a certain system
the internal energy U is related to the entropy S, particle number
N, and volume V through
where d is a constant.
(a) Show that the system satisfies the ideal gas law
PV=NkT
independent of
the value of the constant d.
(b) Find the coefficient 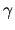 in the
adiabatic equation of
state
in the
adiabatic equation of
state
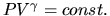 and the
molar specific heats CP and CV of the system.
and the
molar specific heats CP and CV of the system.
2.
If the ratio of oxygen to nitrogen is 1/4 at sea level, what
would you expect the ratio to be at an altitude of 5000m. Assume
an isothermal atmosphere and temperature T=0oC.
3:
A sealed cylindrical vessel is in contact with a heat bath at constant
temperature T. A friction-less airtight piston of weight mg divides
the container into two volumes V1 and
V2=V-V1. There are N1
ideal gas atoms in the top partition and N2 in the bottom partition.
The area of the piston is A.
(a). Find the equilibrium height of the piston.
(b). The ideal gas is replaced by a real single component
gas. At a certain temperature the bottom partition is found to contain
a puddle of liquid coexisting with its vapor. Which of the following
statements may be true at equilibrium:
(i). The top partition contains a liquid in coexistence
with its vapor.
(ii). The top partition contains only vapor.
(iii). The top partition contains only liquid.
(c). Assume that the cylinder is turned around so that
the piston moves horizontally and that N1=N2. Which of the three
statements in part (b) may now be true?


Next: About this document ...
Birger Bergersen
2000-11-30