


Next: About this document ...
PHYSICS 313
Problem set 8 2001
OPTIONAL PROBLEM SET
Given November 26
Due December 11 10 a.m. if you want it to count towards your assignments mark.
Problem 1:
It was shown in class that the variance of energy fluctuations at constant temperature
volume and particle number can be written
A typical fluctuation of the energy about its mean energy would be the standard deviation
(the square root of the variance). Calculate the typical fluctuation in energy of
of an air bubble of radius 1 mm at 1 bar and 300 K. What fraction of the total internal energy does this represent?
Problem 2:
The Helmholtz free energy of a diatomic Van der Waals fluid can be written
where 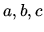 are constants
are constants
a: Calculate the Gibbs free energy by first calculating the pressure using
and substituting into 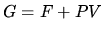
b:The Gibbs free energy can also be computed from
and the Gibbs Duhem relation 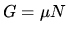 . Check that the two methods give the same result.
. Check that the two methods give the same result.
c: What is the internal energy 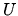 of the Van der Waals fluid?
of the Van der Waals fluid?
Problem 3:
a: The latent heat for boiling 1 kg of water at atmospheric pressure is
540 kCal, how much of this represents the change in internal energy, how much is work done
in expanding the vapor? The volume of liquid water can be considered to be negligible
in this problem.
b: A pressure cooker has a safety valve that releases vapor to the atmosphere when the pressure reaches 2 atm. Estimate at what temperature water will boil in this pressure cooker.
Problem 4:
A room is maintained at of temperature of 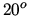 C when the outside temperature is
C when the outside temperature is 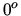 C.
What is the relative costs of heating the room with electricity (100% efficiency) and an
ideal heat pump? Take into account that in order to maintain a reasonable power output the heat pump is not used to heat the room directly, but to heat water to
C.
What is the relative costs of heating the room with electricity (100% efficiency) and an
ideal heat pump? Take into account that in order to maintain a reasonable power output the heat pump is not used to heat the room directly, but to heat water to 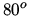 C. The water is then circulated to warm the room, keeping it at
C. The water is then circulated to warm the room, keeping it at 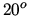 C.
C.
Problem 5:
The surface tension of water at 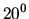 C is 0.073 J/m
C is 0.073 J/m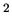 . What is the difference
in chemical potential of water molecules in bulk water and in a droplet of radius 1
. What is the difference
in chemical potential of water molecules in bulk water and in a droplet of radius 1 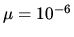 m?
Is the chemical potential in the droplet higher or lower than for bulk water?
m?
Is the chemical potential in the droplet higher or lower than for bulk water?



Next: About this document ...
Birger Bergersen
2001-12-11