


Next: About this document ...
PHYSICS 313
Problem set 5 2001
Given October 22
Due Oct 31
Problem 1:
A volume 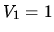 m
m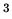 of air is initially at
of air is initially at 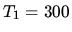 K, and the pressure is
K, and the pressure is 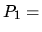 1 bar. Assume air to be an ideal gas with
1 bar. Assume air to be an ideal gas with 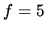 .
The gas is compressed until the volume is
.
The gas is compressed until the volume is 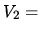 one half and the
pressure is
one half and the
pressure is 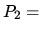 twice the initial value, along a straight line in a
twice the initial value, along a straight line in a 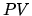 diagram.
Along this line we can write for the pressure
diagram.
Along this line we can write for the pressure
a:
Show that if the volume changes by 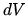 along the straight line segment the
heat provided to the gas will be
along the straight line segment the
heat provided to the gas will be
b:
During which part of the compression will heat be provided to the gas?
During which part will the gas expel heat?
c:
Is the point along the straight line segment where
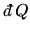 changes sign the same as
where the temperature is a maximum?
changes sign the same as
where the temperature is a maximum?
Problem 2:
In the "Sargent" ideal gas cycle n mols of the gas is initially at temperature 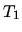 .
The gas is then compressed adiabatically until the temperature is
.
The gas is then compressed adiabatically until the temperature is 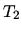 .
It is then heated at constant volume until the temperature is
.
It is then heated at constant volume until the temperature is 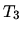 .
It is next expanded adiabatically until the temperature is
.
It is next expanded adiabatically until the temperature is 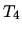 . Finally
the cycle is completed by a compression at constant pressure until the
temperature returns to
. Finally
the cycle is completed by a compression at constant pressure until the
temperature returns to 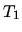 .
.
a:
What is the heat 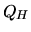 provided to the gas.
provided to the gas.
b:
What is the waste heat 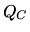 given up by the gas.
given up by the gas.
c:
What is the net work done by the system
d:
Assume that
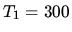 K,
K, 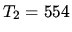 K,
K, 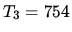 K,
K, 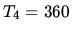 K.
What is the thermodynamic efficiency
K.
What is the thermodynamic efficiency 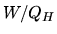 ?
?



Next: About this document ...
Birger Bergersen
2001-11-02