


Next: About this document ...
PHYSICS 313
Problem set 4 2001
Given October 15
Due Oct 24
Problem 1:
In the model of a polymer considered in class the entropy was found to be
where 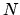 is the total number of links between monomers and
is the total number of links between monomers and 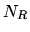 the number of links in the direction of an applied force. The stretched length
the number of links in the direction of an applied force. The stretched length 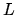 is
is
a:
Express the entropy as a function of the thermodynamic variables 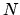 and
and 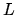 and derive a formula for the force required to stretch the polymer to length
and derive a formula for the force required to stretch the polymer to length 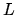 using
using
b:
A mass M is hanging by a rubber band from a fixed support.
If the simple model above is correct, will the mass move up or down if the
temperature is incresed?
c: Find the chemical potential per monomer of a polymer chain of
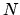 links and stretched length
links and stretched length 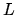 .
.
Problem 2:
a:
A monatomic ideal gas is at constant temperature and in a gravitational
field 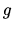 . The internal energy per particle at height
. The internal energy per particle at height 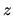 is
is
Use the Sackur Tetrode equation for entropy and
to finde a formula for 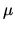 .
.
b:
Show that for the chemical potential to stay constant the pressure decreases with height according to the barometric
formula
c:
For the chemical potential of a monatomic ideal gas to stay
constant while the temperature decreases will the density 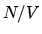 (and
hence the pressure) have to increase or decrease?
(and
hence the pressure) have to increase or decrease?
d:
In the atmosphere the temperature tends to decrease with altitude.
Does this mean that the barometric formula
over-estimates or under-estimates the pressure at high altitudes?



Next: About this document ...
Birger Bergersen
2001-10-22