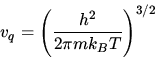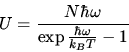Next: About this document ...
PHYSICS 313
Midterm October 10 2001.
Answer all three questions. Allowed aids 1 double-sided formula sheet. Calculator.
Problem 1:
A cylinder contains two chambers each with 1 mol of air initially
at 300 K and pressure 1 bar. The chambers are separated by an airtight piston which moves horizontally without friction. The left chamber is heated until
the volume on the right hand side is one half of what it was initially.
The right chamber is kept at constant temperature from heat flowing in and out from the outside, but the piston is thermally insulated so that no heat flows between the two chambers.
a:
What is the pressure after the compression?
b:
What is the temperature in the left chambers after the compression?
c:
How much work was done on the right chamber?
d:
How much heat was provided to the two chambers?
Problem 2:
a:
What is the change in entropy of 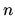 mols of a monatomic ideal gas,
if the pressure doubles at constant volume?
mols of a monatomic ideal gas,
if the pressure doubles at constant volume?
b:
What is the change in entropy of 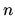 mols of a monatomic ideal gas change
if the pressure doubles at constant temperature?
mols of a monatomic ideal gas change
if the pressure doubles at constant temperature?
Problem 3:
Find the entropy per oscillator of an Einstein solid at the Einstein temperature?
Some formulas:
In the formulas
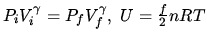 , we have
, we have
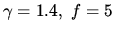 for air, while
for air, while
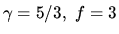 for a monatomic ideal gas.
for a monatomic ideal gas.
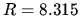 J mol
J mol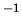 K
K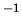 in ideal gas law
in ideal gas law 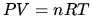 .
.
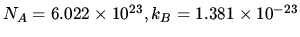 J K
J K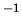 .
.
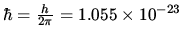 J s
J s
1 atm 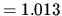 bar
bar
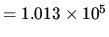 N m
N m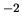 .
The Sackur Tetrode formula for the entropy of a monatomic ideal gas
is
.
The Sackur Tetrode formula for the entropy of a monatomic ideal gas
is
For Einstein solid



Next: About this document ...
Birger Bergersen
2002-10-05