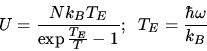Next: About this document ...
PHYSICS 313
Midterm October 1 2003.
Answer all questions. Allowed
aids 1 double-sided formula sheet. Calculator.
Problem 1:
One mol of an ideal gas undergoes the following processes.
A Heating at constant volume from a state 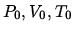 to one in which
the pressure is
to one in which
the pressure is 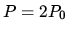 .
.
B Expansion at constant temperature until 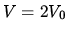 .
.
C Cooling at constant volume until the temperature returns to 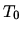 .
.
D Compression at constant temperature until 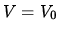 .
.
a: Draw a 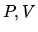 diagram for the process.
diagram for the process.
b: Using the sign convention for the fist law of thermodynamics
list the sign of the heat 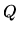 and work
and work 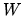 at the four stages of the process.
at the four stages of the process.
c: Calculate 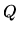 and
and 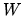 for each of the four steps.
for each of the four steps.
Problem 2:
A hot air balloon has a volume of 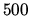 m
m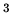 . The temperature of the air inside is 450 K.
The outside temperature is T=300 K. At ground level the pressure is 1 bar.
The mass of one mol of air is
. The temperature of the air inside is 450 K.
The outside temperature is T=300 K. At ground level the pressure is 1 bar.
The mass of one mol of air is 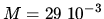 kg mol
kg mol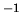 .
.
a: What is the mass of the air inside the balloon?
b: What is the maximum mass of the balloon plus payload, excluding the air inside, for the balloon to be
able to rise. Neglect the volume of the "skin" of the balloon and its payload.
Problem 3:
Consider an
Einstein solid at the Einstein temperature
a: How many vibrational quanta will there be per oscillator on the average?
b: What is the entropy per oscillator?
Some
formulas: 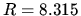 J mol
J mol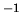 K
K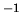
Ideal gas: 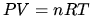
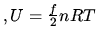
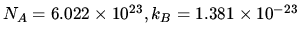 J K
J K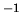 .
.
1 atm 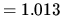 bar
bar
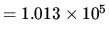 N m
N m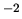 .
.
For Einstein solid



Next: About this document ...
Birger Bergersen
2003-10-10