Next: About this document ...
PHYSICS 313
Midterm October 4 2002.
Answer all questions. Allowed
aids 1 double-sided formula sheet. Calculator.
Problem 1:
A cylinder contains two chambers each of 10 litres (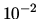 m
m ).
One chamber contains 1 mol of a monatomic gas. The other chamber is
empty (vacuum). The temperature is 300 K.
).
One chamber contains 1 mol of a monatomic gas. The other chamber is
empty (vacuum). The temperature is 300 K.
a:. What is the initial pressure in the chamber containing the gas?
b:. The wall separating the two chambers is ruptured.
The gas
expands freely into the initially empty chamber. What is now the
temperature and pressure of the gas filling both chambers?
c:. A piston is used to compress quickly (adiabatically) the 20 liter volume of gas to 10
liters. What is then pressure and temperature?
d:. Why are your answers in a: and c: different
(they better be!)?
Problem 2:
Two glass bulbs each of 1 litre, are connected by a capillary (thin) tube.
The apparatus is sealed and contains air at a pressure of 1 bar and 300 K.
What will the pressure become if one bulb is kept at 300 K,
but the other bulb is heated to 400 K? Assume that the volume of the
connecting tube is negligible, but the air can flow between the two
bulbs so the pressure is the same in both. Neglect any thermal expansion
of the bulbs.
Problem 3:
Consider an
Einstein solid at twice the Einstein temperature
a: How many vibrational quanta will there be per oscillator?
b: What is the entropy per oscillator?
c: What is the specific heat per mol?
Some
formulas:
In the formulas
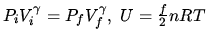 ,
we have
,
we have
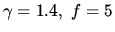 for air, while
for air, while
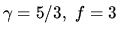 for a monatomic
ideal gas.
for a monatomic
ideal gas.
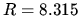 J mol
J mol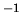 K
K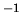 in ideal gas law
in ideal gas law 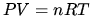 .
.
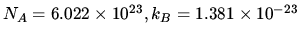 J K
J K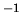 .
.
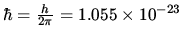 J s
J s
1 atm 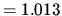 bar
bar
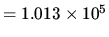 N m
N m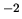 .
.
For Einstein solid



Next: About this document ...
Birger Bergersen
2002-10-09