


Next: About this document ...
PHYSICS 313
Sessional Examination,
December 18 2000
Answer 4 out of the 5 problems. All problems have equal
value. If all 5 problems are attempted credit will be given for the best 4 answers.
A number of potentially useful formulas are given at the end of the paper. Time 2 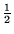 hours. Allowed aids two sheets of notes (4pages), calculator.
hours. Allowed aids two sheets of notes (4pages), calculator.
Problem 1:
a:
The heat capacity at constant pressure for a mole of 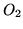 is
is
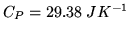 at room temperature. What is the thermodynamic number of degrees of freedom
at room temperature. What is the thermodynamic number of degrees of freedom 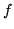 at this temperature?
at this temperature?
b:
Give a physical interpretation of this result.
c: Answer the same questions for a mole of 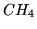 for which
for which 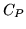 is
is
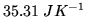 at room temperature.
at room temperature.
Problem 2:
a:
A container contains a monatomic ideal gas with 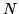 atoms at temperature
atoms at temperature 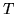 in a
volume
in a
volume 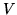 . We now halve the number of particles keeping
. We now halve the number of particles keeping 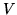 and
and 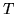 constant. What is the change in the chemical potential? (A formula
for the chemical potential of a monatomic ideal gas is given at the end of the exam).
constant. What is the change in the chemical potential? (A formula
for the chemical potential of a monatomic ideal gas is given at the end of the exam).
b:
We didn't derive a formula for the chemical potential when the gas molecules have
internal degrees of freedom, but let us assume that the chemical potential of a diatomic
gas is
where c is a constant. A diatomic ideal gas, originally with N molecules, temperature T
and volume V, is heated at constant N,V so that the temperature is doubled. What is
the change in the chemical potential?
c:
The inner surface of the container contains absorption sites to which gas molecules
can stick. Assume that the probability that a given absorption site is occupied
is
where 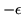 is the binding energy to the site and
is the binding energy to the site and 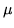 is the chemical potential of
the gas. Assume that at a given pressure the probability that a site is occupied is 0.5.
What is the probability if one half of the molecules are pumped out of the container keeping
is the chemical potential of
the gas. Assume that at a given pressure the probability that a site is occupied is 0.5.
What is the probability if one half of the molecules are pumped out of the container keeping
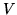 and
and 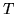 constant? Does it matter if the gas is monatomic or diatomic?
constant? Does it matter if the gas is monatomic or diatomic?
Problem 3:
A van der Waals fluid satisfies the equation of state
and the critical points occurs at
The compression factor of a fluid is defined as
the ratio
a:
Find the compression factor for a van der Waals fluid at the critical point. Show
that this quantity is independent of the constants 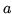 and
and 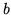 .
.
b:
For water at the critical point 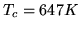 ,
, 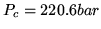 and the compression ratio
is 0.274. Find
and the compression ratio
is 0.274. Find 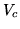 for 1 mole of water in
for 1 mole of water in 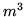 .
.
c:
You can fit the constants 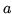 and
and 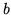 to any of two of the three quantities
to any of two of the three quantities 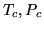 and
and 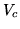 . The third quantity will then deviate from its true value. What are the values of
. The third quantity will then deviate from its true value. What are the values of 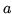 and
and 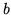 in SI units if you fit to
in SI units if you fit to 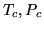 ?
to
?
to 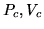 ?
?
Problem 4:
A car battery contains 6 lead acid cells. Each cell runs on the reaction
We have
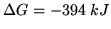 ,
,
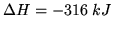 per mole for the reaction
at
per mole for the reaction
at 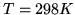 .
At -electrode 2 electrons are given up to the solution while 2 electrons are
picked up at the +electrode for each time the reaction occurs.
.
At -electrode 2 electrons are given up to the solution while 2 electrons are
picked up at the +electrode for each time the reaction occurs.
a:
What is the voltage of the battery?
b:
Is excess heat produced when the battery is charged or when it is discharged?
Justify your answer.
c:
Estimate how the voltage changes if the temperature is increased to 320 K.
Problem 5:
A heat pump is a device which heats a volume, e.g. a building, by pumping in heat from the cold outside. Let 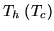 be the inside (outside) temperature,
be the inside (outside) temperature, 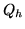 the heat provided and
the heat provided and 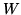 the work needed to provide the heat. The coefficient
of performance is
the work needed to provide the heat. The coefficient
of performance is 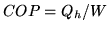 .
.
a:
Use the second law of thermodynamics to derive an upper limit to the
achievable COP.
b:It is 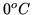 outside and
outside and 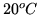 inside. How many kilowatt hours of
electric energy would be the minimum required to produce a kW hour of heat,
using a heat pump? What would be the answer if it is
inside. How many kilowatt hours of
electric energy would be the minimum required to produce a kW hour of heat,
using a heat pump? What would be the answer if it is 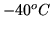 outside?
outside?
c: In the summer a heat pump be run in reverse to perform as an air conditioner.
How many kW hours of work would be the minimum required to remove one
kW hour of heat from the building, if it is 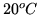 inside and
inside and 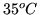 outside.
outside.
-------------------
Some formulas:
Chemical potential of monatomic ideal gas:
Gibbs free energy:
Helmholtz free energy:
Differential form of second law:
Heat capacity/mole at constant volume of ideal gas
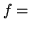 number of thermodynamic degrees of freedom/molecule.
number of thermodynamic degrees of freedom/molecule.
Specific heat at constant pressure of ideal gas
Some constants
Return to title page



Next: About this document ...
Birger Bergersen
2004-03-08
![]() is
is
![]() at room temperature. What is the thermodynamic number of degrees of freedom
at room temperature. What is the thermodynamic number of degrees of freedom ![]() at this temperature?
at this temperature?
![]() for which
for which ![]() is
is
![]() at room temperature.
at room temperature.
![]() atoms at temperature
atoms at temperature ![]() in a
volume
in a
volume ![]() . We now halve the number of particles keeping
. We now halve the number of particles keeping ![]() and
and ![]() constant. What is the change in the chemical potential? (A formula
for the chemical potential of a monatomic ideal gas is given at the end of the exam).
constant. What is the change in the chemical potential? (A formula
for the chemical potential of a monatomic ideal gas is given at the end of the exam).
![]() be the inside (outside) temperature,
be the inside (outside) temperature, ![]() the heat provided and
the heat provided and ![]() the work needed to provide the heat. The coefficient
of performance is
the work needed to provide the heat. The coefficient
of performance is ![]() .
.
![]() outside and
outside and ![]() inside. How many kilowatt hours of
electric energy would be the minimum required to produce a kW hour of heat,
using a heat pump? What would be the answer if it is
inside. How many kilowatt hours of
electric energy would be the minimum required to produce a kW hour of heat,
using a heat pump? What would be the answer if it is ![]() outside?
outside?
![]() inside and
inside and ![]() outside.
outside.
![\begin{displaymath}\mu=-kT\ln\left[\frac{V}{N}\left(\frac{2\pi mkT}{h^2}\right)^{3/2}\right]\end{displaymath}](img38.gif)