


Next: About this document ...
PHYSICS 313
Sessional exam,
December 10 2002
Answer 4 out of the 5 problems. All problems have equal
value. If all 5 problems are attempted credit will be given for the best 4 answers.
A number of potentially useful formulas are given at the end of the paper. Time 2 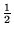 hours. Allowed aids three sheets of notes (6 pages), calculator.
hours. Allowed aids three sheets of notes (6 pages), calculator.
Problem 1:
An imperfect gas satisfies the van der Waals equation of state. The Helmholtz free energy can be written
where 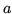 and
and 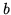 are constants and
are constants and
a: Find the entropy of such a gas with 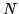 particle at temperature
particle at temperature 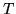 , volume
, volume 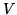 .
.
b: What is the heat capacity 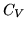 , under the conditions of a: ?
, under the conditions of a: ?
c: The gas is expanded from volume 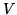 to 2
to 2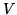 , keeping the temperature constant.
, keeping the temperature constant.
- What is the change in internal energy during the expansion?
- How much work is done by the gas during the expansion?
Problem 2:
In a hydrogen fuel cell the chemical reactions are
- At negative electrode
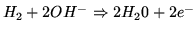
- At positive electrode
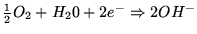
- The net result is
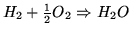 .
.
Some thermodynamic properties per mol of the constituents
at 298 K, 1 bar are listed below
| Substance |
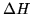 (kJ) (kJ) |
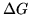 (kJ) (kJ) |
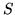 (JK (JK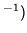 |
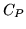 (JK (JK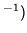 |
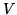 (cm (cm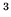 ) ) |
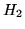 |
0 |
0 |
130.68 |
28.82 |
|
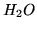 |
-285.83 |
-237.13 |
69.91 |
75.29 |
18.068 |
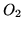 |
0 |
0 |
205.14 |
29.38 |
|
a: What is the voltage of the cell?
b: What is the change in the Gibbs free energy for the constituents if the pressure
of the oxygen, hydrogen and water each are increased
from 1 bar to 10 bar. The temperature is kept constant at 298 K. Neglect any volume change of the liquid
water with pressure.
c: How much will the voltage of the cell increase or decrease after the pressure has been increased?
Problem 3:
You have a system in which particles can occupy any one of two energy levels with energy
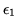 and
and 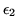 respectively. Assume that there are two particles in the system
and that the temperature is given by
respectively. Assume that there are two particles in the system
and that the temperature is given by 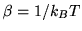 . Find the probability that any one particle
is in state 1 and the other in 2 if
. Find the probability that any one particle
is in state 1 and the other in 2 if
a: The particles are distinguishable.
b: The particles are identical and two particles are forbidden from occupying the
same state (fermions).
c: The particles are identical and several particles are allowed to occupy the
same state (bosons).
Problem 4:
One mole of a monatomic ideal gas is initially at 300 K and 1 bar. The system then undergoes the following
cyclic sequence of processes:
- Heating at constant volume until the temperature is 400 K.
- Adiabatic expansion until the temperature returns to 300 K.
- Isothermal compression until the volume returns to its initial value.
a: Draw a PV diagram for the cycle?
b: Will the system work as an engine or a heat pump? Justify your answer!
c: Work out the heat and work for each step of the cycle. Specify if the heat and work is added to the system
or taken from it.
Problem 5:
The temperature and of air at ground level is 293 K. The saturation vapor pressure
at that temperature is 0.023 bar and the actual partial pressure 50% of that (50% relative humidity). The latent heat of evaporation of water around that temperature is 44 KJ/mol. Assume that the temperature drops at a rate of
1 K per 100 m.
a: Neglect the pressure drop with altitude and estimate at what altitude will the saturation water
pressure be exceeded, and a cloud forms.
b: Not only the temperature, but also the pressure will drop with altitude. Estimate how much
the vapor pressure has dropped at the altitude you calculated under a:.
-------------------
Some formulas:
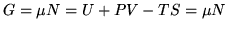 Gibbs free energy
Gibbs free energy
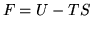 Helmholtz free energy
Helmholtz free energy
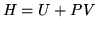 Enthalpy.
Enthalpy.
Differential forms
Heat capacity/mol at constant volume of ideal gas
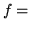 number of thermodynamic degrees of freedom/molecule, with
number of thermodynamic degrees of freedom/molecule, with
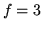 for monatomic gas,
for monatomic gas, 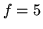 for air.
for air.
Clausius Clapeyron equation
approximate formula for vapor pressure
Adiabatic process
Some constants
The mass of a water molecule is
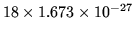 kg.
kg.
The charge of an electron is
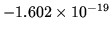 Coulomb.
Coulomb.



Next: About this document ...
Birger Bergersen
2003-01-03