


Next: About this document ...
PHYSICS 313
Sessional exam,
December 13 2001
Answer 4 out of the 5 problems. All problems have equal
value. If all 5 problems are attempted credit will be given for the best 4 answers.
A number of potentially useful formulas are given at the end of the paper. Time 2 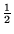 hours. Allowed aids three sheets of notes (6 pages), calculator.
hours. Allowed aids three sheets of notes (6 pages), calculator.
Problem 1:
a:
Which of the following statements is most likely to be correct?
The heat capacity 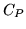 at constant pressure for a mole of
at constant pressure for a mole of 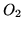 is
is
- 1
-
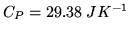
- 2
-
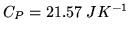
- 3
-
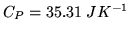
Justify your answers!
b:For an ideal gas 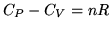 where
where 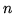 is the number of moles of gas. This implies that
is the number of moles of gas. This implies that
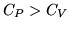 . Explain qualitatively why
. Explain qualitatively why 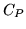 must be greater than
must be greater than 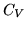 for an ideal gas.
for an ideal gas.
Problem 2:
The Free energy of Black body radiation is
where 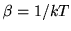 ,
, 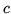 is the speed of light and
is the speed of light and 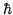 is Planck's constant divided by
is Planck's constant divided by 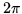 .
Derive formulas for
.
Derive formulas for
a: The entropy and pressure of black-body radiation.
b: The internal energy and enthalpy.
c: Check that the Gibbs free energy 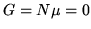 for black body radiation.
Give a physical reason that the chemical potential has to be zero in this case.
for black body radiation.
Give a physical reason that the chemical potential has to be zero in this case.
Problem 3:
a:
An imperfect gas obeys the equation of state
where 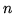 is the number of moles of gas. For this gas
is the number of moles of gas. For this gas 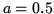 J m
J m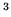 mole
mole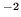 and
and
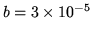 m
m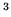 mole
mole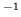 .
What is the work required to compress 0.2 mole of this gas from
.
What is the work required to compress 0.2 mole of this gas from 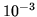 m
m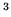 to
to 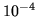 m
m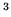 at
at 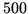 K. Express your result as a formula before
substituting numbers.
K. Express your result as a formula before
substituting numbers.
b:
If the same process had taken place for an ideal gas the work done in compressing the gas
would equal to the heat expelled to keep the temperature constant. For the imperfect
gas this will not be true. Explain why.
Problem 4:
When lead is melted at the pressure of 1 bar the melting point is
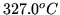 , the density decreases from
, the density decreases from 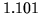 to
to
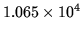 kgm
kgm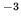 The latent heat of melting is
The latent heat of melting is 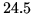 kJ kg
kJ kg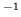 . How much will the
melting temperature change if the pressure is increased to 100 bar. Derive a
formula for the temperature change before substituting numbers. Is the melting temperature
increasing or decreasing?
. How much will the
melting temperature change if the pressure is increased to 100 bar. Derive a
formula for the temperature change before substituting numbers. Is the melting temperature
increasing or decreasing?
Problem 5:
A heat pump is a device which heats a volume, e.g. a building, by pumping in heat from the cold outside. Let 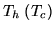 be the inside (outside) temperature,
be the inside (outside) temperature, 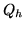 the heat provided and
the heat provided and 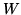 the work needed to provide the heat. The coefficient
of performance is
the work needed to provide the heat. The coefficient
of performance is 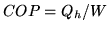 .
.
a:
Use the second law of thermodynamics to derive an upper limit to the
achievable COP.
b:A building is heated by a heat pump which may be assumed to have an ideal
performance. The pump consumes a constant power 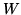 and looses heat to its surroundings
at the rate
and looses heat to its surroundings
at the rate
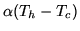 . What temperature will the building reach if the outside
temperature is constant at
. What temperature will the building reach if the outside
temperature is constant at 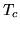 ?
?
-------------------
Some formulas:
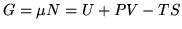 Gibbs free energy
Gibbs free energy
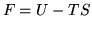 Helmholtz free energy
Helmholtz free energy
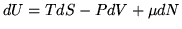 Differential form of second law
Differential form of second law
Heat capacity/mole at constant volume of ideal gas
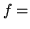 number of thermodynamic degrees of freedom/molecule.
number of thermodynamic degrees of freedom/molecule.
Clausius Clapeyron equation
Some constants



Next: About this document ...
Birger Bergersen
2002-11-28