


Next: About this document ...
PHYSICS 313
Problem set 6 2002
Given Nov 18
Due Nov 27
Problem 1:
The latent heat of melting ice is 80 Kcal kg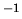 at atmospheric
pressure. The melting temperature is 273 K and the the
density of ice is 917 kg m
at atmospheric
pressure. The melting temperature is 273 K and the the
density of ice is 917 kg m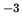 , while the density of water
can be taken to be 1000 kg m
, while the density of water
can be taken to be 1000 kg m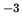 . Estimate the change
in melting temperature if the pressure is increased to 100 bar.
. Estimate the change
in melting temperature if the pressure is increased to 100 bar.
Problem 2:
a: Estimate the difference in chemical potential 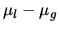 of the gas and liquid phases of water at 300K if the relative humidity is 110%.
of the gas and liquid phases of water at 300K if the relative humidity is 110%.
b: Estimate the radius of a water droplet for which the
excess Gibbs free energy
has a maximum. (Droplets above this size will grow in pure air, while smaller droplets will evaporate.)
Problem 3:
a:
The entropy at oxygen gas at 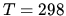 K and atmospheric pressure is 205.14 J K
K and atmospheric pressure is 205.14 J K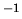 mol
mol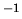 . Estimate the chemical potential of oxygen under these conditions.
. Estimate the chemical potential of oxygen under these conditions.
b: The surface of a container with oxygen at 298 K, 1 atm. pressure
contains discrete absorption sites at which oxygen molecules may be bound.
These sites may be either occupied by an oxygen molecule or empty.
Assume that there are N such sites on the surface, that the internal energy
of an occupied site is 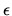 , that the internal energy of an empty site
is zero and that the fraction of sites that are occupied is
, that the internal energy of an empty site
is zero and that the fraction of sites that are occupied is 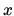 .
Find the entropy of the absorption sites.
.
Find the entropy of the absorption sites.
c: What is the chemical potential of the absorbed oxygen under the conditions in part b?
d: Assume that the chemical potentials of absorbed and gas
phase oxygen are the same and that 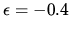 electron volt.
What is the fraction
electron volt.
What is the fraction 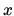 of sites that are occupied?
of sites that are occupied?



Next: About this document ...
Birger Bergersen
2002-11-29