


Next: About this document ...
PHYSICS 313
Problem set 5 2002
Given Oct 18
Due Oct 28
Problem 1:
It is found that for a certain substance the internal energy
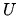 depends on the volume
depends on the volume 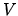 , the number of particles
, the number of particles 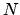 and
the entropy
and
the entropy 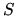 according to
according to
a: Show that the substance satisfies the ideal gas law.
b:Find the coefficient 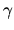 in the adiabatic equation
of state.
in the adiabatic equation
of state.
Problem 2:
A certain device undergoes a thermodynamic cycle where the
working substance can be taken to be an ideal gas with
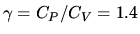 .
The system starts with the pressure
.
The system starts with the pressure 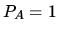 bar, temperature
bar, temperature 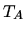 . It is compressed
adiabatically until the pressure is
. It is compressed
adiabatically until the pressure is 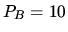 bar. Next it is heated and expands
at constant pressure to temperature
bar. Next it is heated and expands
at constant pressure to temperature 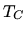 . It next expands adiabatically
until the pressure returns to
. It next expands adiabatically
until the pressure returns to 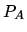 . Finally it is cooled and compressed
at constant pressure until the temperature returns to its original value
. Finally it is cooled and compressed
at constant pressure until the temperature returns to its original value
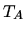 .
.
a: Will the device work as a heat engine or a heat pump?
b: Show that the coefficient of performance is independent
of 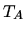 and
and 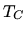 and find the COP.
and find the COP.
Problem 3:
One mol of an ideal gas is mixed with 2 mols of another ideal gas. Initially
the pressure of both gases is 1 bar and the temperature is 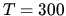 K.
In the final state the pressure and temperature is unchanged.
Find the entropy of mixing.
K.
In the final state the pressure and temperature is unchanged.
Find the entropy of mixing.



Next: About this document ...
Birger Bergersen
2002-10-28