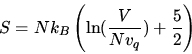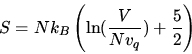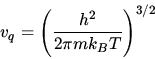Next: About this document ...
PHYSICS 313
Problem set 4 2002
Given Oct 7
Due Oct 16
Problem 1:
Use the Sackur-Tetrode formula
to estimate for which temperature the entropy would turn negative in
a monatomic ideal gas with the atomic mass of He at a pressure of 1 bar?
at a pressure of 1 bar?
Problem 2: Verify using the Sackur-Tetrode formula
that the entropy of a monatomic ideal gas stays constant
under an adiabatic expansion
if 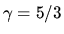 .
.
Problem 3:. Assume that the entropy of a diatomic ideal
gas is of the form of the Sackur Tetrode formula, but with
where the constant depends on molecular properties, and natural
constants such as
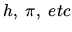 but not on the thermodynamic
variables
but not on the thermodynamic
variables 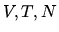 . What is the value of
. What is the value of 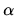 if
if
Birger Bergersen
2002-10-08