


Next: About this document ...
PHYSICS 313
Problem set 4 2003
Given Oct. 17 2003
Due Oct 27 2003
Problem 1:
In class we have generally illustrated thermodynamic cycles by plots
in the 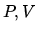 plane. As an alternative one could plot the processes in the
plane. As an alternative one could plot the processes in the
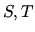 (entropy vs. temperature) plane.
(entropy vs. temperature) plane.
a: Plot the Carnot cycle in the 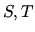 plane.
plane.
b: Plot the Otto cycle in the 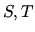 plane
plane
Problem 2:
a:
Use the Sackur-Tetrode formula for the entropy of a monatomic ideal gas
to find a formula for the Helmholtz free energy.
b: Find the chemical potential of Argon at 300 K and pressure 1 bar.
c: How does the chemical potential change if the pressure is increased
to 100 bar.
Problem 3:
When the pressure on a 1 kg liquid is increased isothermally from 1 bar to
3000 bar the Gibbs free energy increases by 360 kJ. Estimate the density
of the liquid.
Birger Bergersen
2003-11-03