


Next: About this document ...
PHYSICS 313
Problem set 3 2003
Given Oct. 8 2003
Due Oct 17 2003
Problem 1:
Argon is a monatomic ideal gas with molecular weight 39.95 g mol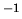 .
.
a: Calculate the entropy of 1 mol of argon at 300 K and pressure
1 bar.
b:The gas in a: is heated to 600 K at constant pressure. What is the change in entropy?
c: The gas in a: is expanded adiabatically until its volume is
doubled. What is the change in entropy?
Problem 2:
The outcome of 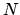 tosses of a fair coin is
tosses of a fair coin is 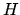 heads and
heads and 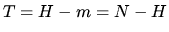 .
The probability of the difference
.
The probability of the difference 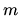 between the number of heads and
tails is to a good approximation a Gaussian with mean
between the number of heads and
tails is to a good approximation a Gaussian with mean
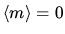 and variance
and variance 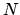 .
a: What is the entropy associated with outcomes
.
a: What is the entropy associated with outcomes 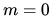 if
if 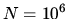 ?
?
b; What is the entropy in the two cases for the outcome 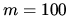 ,
, 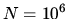 ?
?
Problem 3:
1 kg of aluminum at 400 K is dunked into 1 kg of water at 300K.
Assume that no heat is lost to the outside, that Al is an Einstein solid with
atomic weight 27 g mol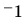 , with an Einstein temperature much less that
300 K, and that the specific heat of water is 1 cal g
, with an Einstein temperature much less that
300 K, and that the specific heat of water is 1 cal g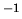
a: What is the final temperature?
b: What is the change in entropy of the water?
c: What is the change in entropy of the aluminum?



Next: About this document ...
Birger Bergersen
2003-10-10