


Next: About this document ...
PHYSICS 313
Problem set 1 2003
Given September 12 2003
Due September 22 2003
Problem 1:
A thermally insulated cylinder is closed at both ends.
The cylinder contains two chambers which are separated by
an airtight frictionless piston, Initially the piston
is clamped in a position where the volume to the left is 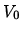 while the volume to the right is 3
while the volume to the right is 3 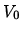 . Both
compartments contain the same monatomic ideal gas.
The initial
temperature is
. Both
compartments contain the same monatomic ideal gas.
The initial
temperature is 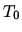 on both sides, while the pressure
is
on both sides, while the pressure
is 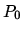 to the left and 2
to the left and 2 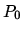 to the right.
The piston is then released and after some motion
back and forth comes to rest.
What is the final temperature, volume and pressure
on each side if the piston is a thermal conductor
(so that the temperature ends up the same on both sides).
to the right.
The piston is then released and after some motion
back and forth comes to rest.
What is the final temperature, volume and pressure
on each side if the piston is a thermal conductor
(so that the temperature ends up the same on both sides).
Problem 2:
An ideal gas of volume 1 liter and pressure 10 bar undergoes
a quasistatic adiabatic expansion until the pressure drops to
1 bar.
Assume 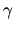 to be 1.4
to be 1.4
a: What is the final volume?
b: If the initial temperature was 300 K what is
the final temperature?
Problem 3:
Two glass bulbs of the same volume contains dry air.
They are connected by a thin tube of negligible volume.
Initially the temperature 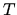 is the same on both sides.
The left bulb is then kept at temperature
is the same on both sides.
The left bulb is then kept at temperature 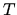 while the
right bulb is heated. Assume that since the bulbs are connected
the two sides always have the same pressure. To what temperature
must the temperature on the right side be increased for the
pressure inside the assembly to double.
while the
right bulb is heated. Assume that since the bulbs are connected
the two sides always have the same pressure. To what temperature
must the temperature on the right side be increased for the
pressure inside the assembly to double.



Next: About this document ...
Birger Bergersen
2003-09-12