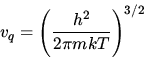Next: About this document ...
PHYSICS 313
Second midterm November 6 2002.
Answer all three questions. Allowed aids 1 double-sided formula sheet. Calculator.
Some potentially useful formulas are given on the second page of the exam.
Problem 1:
A container has initially 2 compartments each with volume 1 liter. One compartment contains 1 mol and the other 2 mol of the same monatomic ideal gas as the first compartment. The temperature is initially 300K. The wall separating the two compartments is removed.
a: What is the final pressure and temperature?
b: What is the change in entropy when the gases in the two containers are mixed?
c:Would you have got a different result in b: if the gas had been diatomic?
Problem 2:
A refrigerator pumps heat from the inside compartment into the room in which it is located.
Let 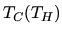 be the inside (outside) temperature,
be the inside (outside) temperature, 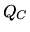 the heat remove and
the heat remove and 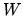 the work needed to do this. The coefficient of performance is
the work needed to do this. The coefficient of performance is 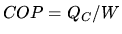 .
.
a: Use the second law of thermodynamics to derive an upper limit to the achievable  .
.
b: An actual fridge operates at 1/2 the ideal performance. It consumes constant power  and heat seeps into the cold compartment at the rate
and heat seeps into the cold compartment at the rate
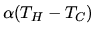 , with
, with 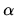 a constant. What temperature will be reached inside the fridge if the temperature
a constant. What temperature will be reached inside the fridge if the temperature 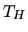 is constant?
is constant?
Problem 3: The entropy of liquid water at 298 K atmospheric pressure per mol is
S=69.91 JK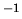 .
.
a: What is the change in entropy per mol if water is heated from 298 K to 340 K at constant pressure.
b: Estimate the change in the Gibbs free energy when 1 mol water is heated from 298 K to 340 K at constant pressure.
Some formulas:
In the formulas
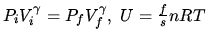 , we have
, we have
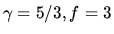 for a monatomic gas, while
for a monatomic gas, while
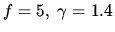 for air.
for air.
Sackur tetrode formula for monatomic ideal gas
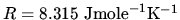 in ideal gas law
in ideal gas law 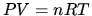 .
.
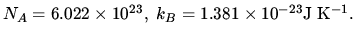
1 atm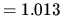 bar
bar
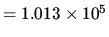 N m
N m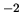 .
.
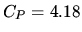 kJ kg
kJ kg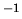 for water.
for water.



Next: About this document ...
Birger Bergersen
2002-11-19